Mechanism of action
SUNOSI is the first and only DNRI approved for the treatment of excessive daytime sleepiness (EDS) in OSA1
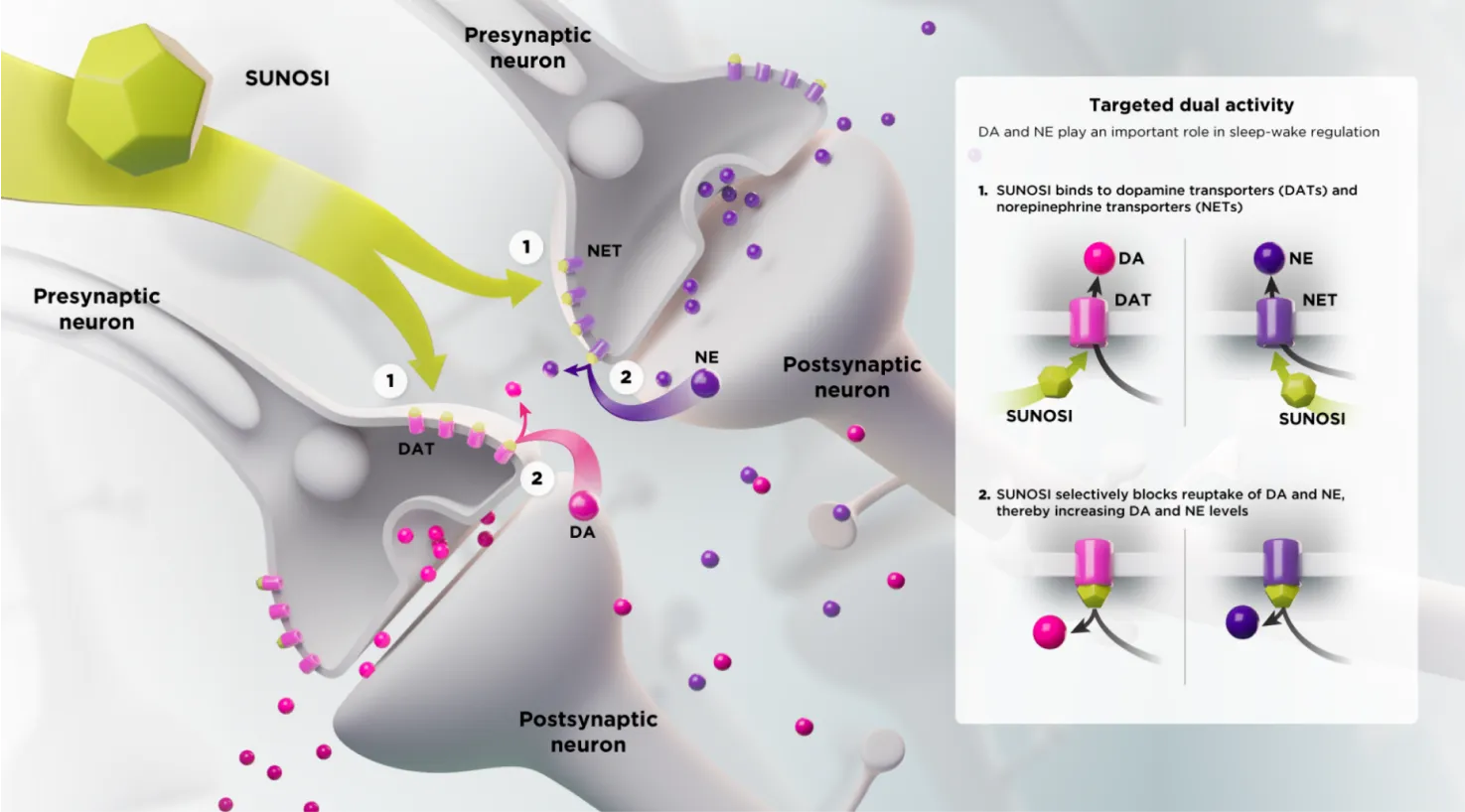
SUNOSI is thought to work by selectively inhibiting the reuptake of dopamine (DA) and norepinephrine (NE).1,2*
SUNOSI is not a stimulant1,2
DNRI=dopamine-norepinephrine reuptake inhibitor; MOA=mechanism of action; OSA=obstructive sleep apnea.
*As seen in preclinical studies. The exact mechanism of action of SUNOSI is unknown.1,2
Pharmacodynamic Profile
Preclinical binding affinity studies have shown the following1,2:
GABA=gamma-aminobutyric acid.
Select characteristics of wake-promoting agents
There are no head-to-head studies comparing SUNOSI to modafinil or armodafinil. A conclusive comparison should not be drawn regarding efficacy or safety.
SUNOSI1,5 | Modafinil and Armodafinil3-5 | |
|---|---|---|
Proposed MOA | Dopamine and norepinephrine reuptake inhibitor | Dopamine reuptake inhibitor |
DEA Scheduling | Schedule IV: drugs with low potential for abuse and low risk of dependence | Schedule IV: drugs with low potential for abuse and low risk of dependence |
Absorption (Tmax) | 2 hours | Modafinil: 2-4 hours |
Metabolism | Minimally metabolized | Modafinil: Liver via amide hydrolysis and CYP450 |
Protein Binding | 13.3% to 19.4% | Modafinil: ~60%, mainly to albumin |
Excretion | Kidney; approximately 95% of the dose recovered in urine as unchanged solriamfetol | Modafinil: The major route of elimination is metabolism by the liver (~90%) with subsequent renal elimination of the metabolites (<10% as parent compound) |
CYP450 | SUNOSI does not induce or inhibit CYP450. Drug interactions with major CYPs and transporters are not expected in patients taking SUNOSI | Induces CYP3A4 (eg, steroidal contraceptives) |
CYP=cytochrome P450; DEA=Drug Enforcement Administration; MOA=mechanism of action; n/a=not available; Tmax=time to reach peak concentration.
INDICATION AND IMPORTANT SAFETY INFORMATION
INDICATION
SUNOSI is indicated to improve wakefulness in adults with excessive daytime sleepiness (EDS) associated with narcolepsy or obstructive sleep apnea (OSA).
LIMITATIONS OF USE
SUNOSI is not indicated to treat the underlying obstruction in OSA. Ensure that the underlying airway obstruction is treated (e.g., with continuous positive airway pressure (CPAP)) for at least one month prior to initiating SUNOSI. SUNOSI is not a substitute for these modalities, and the treatment of the underlying airway obstruction should be continued.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
SUNOSI is contraindicated in patients receiving concomitant treatment with monoamine oxidase inhibitors (MAOIs), or within 14 days following discontinuation of an MAOI, because of the risk of hypertensive reaction.
WARNINGS AND PRECAUTIONS
Blood Pressure and Heart Rate Increases
SUNOSI increases systolic blood pressure, diastolic blood pressure, and heart rate in a dose-dependent fashion.
Blood Pressure and Heart Rate Increases (Cont’d)
Epidemiological data show that chronic elevations in blood pressure increase the risk of major adverse cardiovascular events (MACE), including stroke, heart attack, and cardiovascular death. The magnitude of the increase in absolute risk is dependent on the increase in blood pressure and the underlying risk of MACE in the population being treated. Many patients with narcolepsy and OSA have multiple risk factors for MACE, including hypertension, diabetes, hyperlipidemia, and high body mass index (BMI).
Assess blood pressure and control hypertension before initiating treatment with SUNOSI. Monitor blood pressure regularly during treatment and treat new-onset hypertension and exacerbations of pre-existing hypertension. Exercise caution when treating patients at higher risk of MACE, particularly patients with known cardiovascular and cerebrovascular disease, pre-existing hypertension, and patients with advanced age. Use caution with other drugs that increase blood pressure and heart rate.
Periodically reassess the need for continued treatment with SUNOSI. If a patient experiences increases in blood pressure or heart rate that cannot be managed with dose reduction of SUNOSI or other appropriate medical intervention, consider discontinuation of SUNOSI.
Patients with moderate or severe renal impairment could be at a higher risk of increases in blood pressure and heart rate because of the prolonged half-life of SUNOSI.
Psychiatric Symptoms
Psychiatric adverse reactions have been observed in clinical trials with SUNOSI, including anxiety, insomnia, and irritability.
Exercise caution when treating patients with SUNOSI who have a history of psychosis or bipolar disorders, as SUNOSI has not been evaluated in these patients.
Patients with moderate or severe renal impairment may be at a higher risk of psychiatric symptoms because of the prolonged half-life of SUNOSI.
Observe SUNOSI patients for the possible emergence or exacerbation of psychiatric symptoms. Consider dose reduction or discontinuation of SUNOSI if psychiatric symptoms develop.
MOST COMMON ADVERSE REACTIONS
The most common adverse reactions (incidence ≥5%) reported more frequently with the use of SUNOSI than placebo in either narcolepsy or OSA were headache, nausea, decreased appetite, anxiety, and insomnia.
Dose-Dependent Adverse Reactions
In the 12-week placebo-controlled clinical trials that compared doses of 37.5 mg, 75 mg, and 150 mg/day of SUNOSI to placebo, the following adverse reactions were dose-related: headache, nausea, decreased appetite, anxiety, diarrhea, and dry mouth.
DRUG INTERACTIONS
Do not administer SUNOSI concomitantly with MAOIs or within 14 days after discontinuing MAOI treatment. Concomitant use of MAOIs and noradrenergic drugs may increase the risk of a hypertensive reaction. Potential outcomes include death, stroke, myocardial infarction, aortic dissection, ophthalmological complications, eclampsia, pulmonary edema, and renal failure.
Concomitant use of SUNOSI with other drugs that increase blood pressure and/or heart rate has not been evaluated, and combinations should be used with caution.
Dopaminergic drugs that increase levels of dopamine or that bind directly to dopamine receptors might result in pharmacodynamic interactions with SUNOSI. Interactions with dopaminergic drugs have not been evaluated with SUNOSI. Use caution when concomitantly administering dopaminergic drugs with SUNOSI.
USE IN SPECIFIC POPULATIONS
Renal Impairment
Dosage adjustment is not required for patients with mild renal impairment (eGFR 60-89 mL/min/1.73 m2). Dosage adjustment is recommended for patients with moderate to severe renal impairment (eGFR 15-59 mL/min/1.73 m2). SUNOSI is not recommended for patients with end stage renal disease (eGFR <15 mL/min/1.73 m2).
ABUSE
SUNOSI contains solriamfetol, a Schedule IV controlled substance. Carefully evaluate patients for a recent history of drug abuse, especially those with a history of stimulant or alcohol abuse, and follow such patients closely, observing them for signs of misuse or abuse of SUNOSI (e.g., drug-seeking behavior).
SUN HCP ISI 05/2022
Please see full Prescribing Information.
REFERENCES:
- SUNOSI (solriamfetol) [prescribing information]. New York, NY: Axsome Therapeutics, Inc.
- Baladi MG, Forster MJ, Gatch MB, et al. Characterization of the neurochemical and behavioral effects of solriamfetol (JZP-110), a selective dopamine and norepinephrine reuptake inhibitor. J Pharmacol Exp Ther. 2018;366(2):367-376.
- NUVIGIL (armodafinil) [prescribing information]. Parsippany, NJ: Teva Pharmaceuticals USA, Inc.
- PROVIGIL (modafinil) [prescribing information]. Parsippany, NJ: Teva Pharmaceuticals USA, Inc.
- Drug Scheduling. United States Drug Enforcement Administration. Accessed January 9, 2025. https://www.dea.gov/drug-scheduling

